Innovative REE extraction from primary and secondary resources using ionic liquids
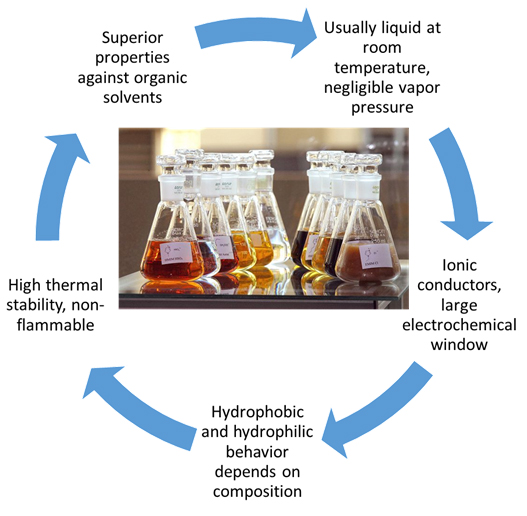
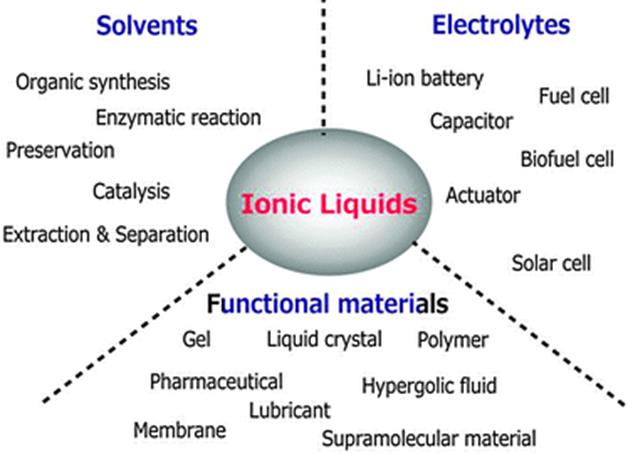
Ionic liquids (ILs) are fluids which consist entirely of ions. They are versatile solvents with applications in many fields such as catalytic reactions, separation processes, and electrochemistry. ILs consist of an organic cation and an inorganic or organic anion, and what makes them so attractive is that the almost infinite cation-anion combinations allow us to tailor IL properties, rendering them as designer solvents. They have many advantages compared to common organic solvents:
- They are usually liquid at room temperature and negligible vapour pressure
- They often have wide liquidus ranges, over temperature intervals greater than that of water
- They are intrinsic ionic conductors, with large electrochemical windows: in some cases more than 4 V, while the electrochemical window of water is 1.23 V
- They have high thermal stability and are non-flammable
- Hydrophobic and hydrophilic behaviour depends on composition
Ionometallurgy is the term coined for mineral and metal processing using ionic liquids. As designer solvents, they can be used in many of the main steps of REE extractive metallurgy. In the EURARE project, ILs are being studied at NTUA and KU Leuven as:
- Lixiviants for low grade REE resources
- Solvents for REE liquid-liquid extraction and separation
- Electrolytes for REE electrodeposition
The application of ionic liquids as an alternative to conventional metallurgy is driven by the need to find more efficient and environmentally-friendly metallurgical processes for REE dissolution-separation and production.
Mineral leaching
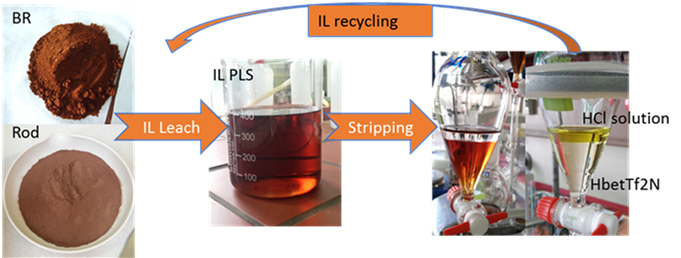
Applying ionic liquids to leach low grade primary and secondary REE resources is a growing area of interest, especially for treating low grade ores from Europe. NTUA has developed an innovative leaching strategy based on the special properties of the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)-imide (HbetTf2N). NTUA's process is capable of:
- Selectively dissolving REE from low grade resources.
- Extracting metals from the generated ionic liquid pregnant solution.
- Producing an acidic solution for further purification.
- Recycling the IL for reuse.
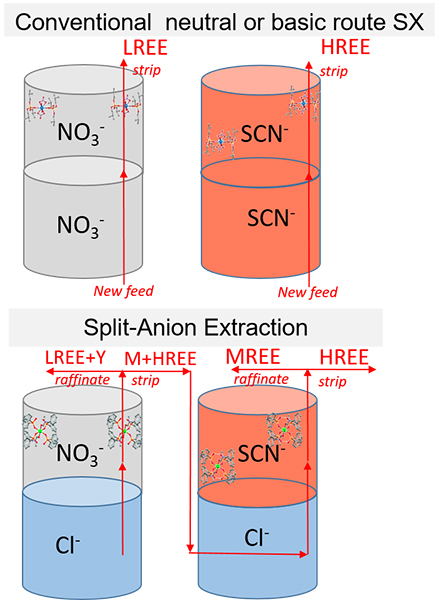
Split-anion extraction
Split-anion extraction is a new method of solvent extraction developed at KU Leuven for the separation of REE, without changing the aqueous phase. The extraction of REE from aqueous chloride feed solutions is carried out using a neutral extractant (Cyanex® 923) dissolved in an ionic liquid (diluent) which contains the REE-coordinating anions. The anion of the ionic liquid (NO3- or SCN-) coordinates and transports the REECl3 from the aqueous phase into the water-immiscible ionic liquid.
Split-anion extraction is an improvement on current technology, regarding health, safety and environmental standards, because:
- The selective separation of REE into groups from chloride aqueous solutions requires less capital expenditure and an easier waste water treatment
- The replacement of organic solvents by non-fluorinated ionic liquids involves a high concentration of coordinating anions, low volatility, non-flammability and no accumulation of static electricity.
- Little to no pH control and easy stripping by water, means less consumption of chemicals, lower operating costs and easier waste water treatment.