Metal production by salt electrolysis
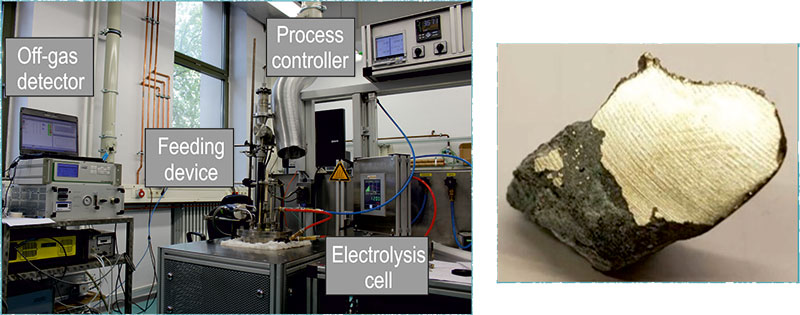
Once the REE have been separated, the remaining step is the electrowinning of high-purity REE metal. Nd and Pr can be produced separately, or as an alloy (didymium), using an oxy-fluoride molten salt electrolysis process. The technology is similar to aluminium electrolysis, but the setup and size differs.
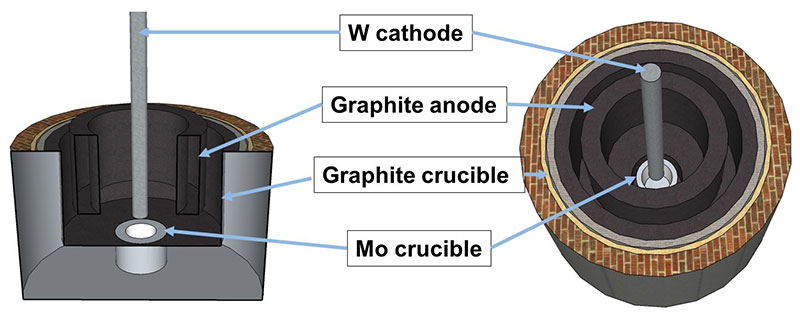
Rare earth oxides (REO) provide the raw material to a fluoride-based electrolyte, which is fed into a closed steel cell operated under inert argon atmosphere. The system contains a hollow carbon ring anode and a tungsten (or molybdenum) cathode, and is heated to around 1050 °C. Oxidation occurs at the anode, where oxygen reacts with carbon to produce CO and CO2 gases. Simultaneously, the reduction reaction occurs on the cathode where the neodymium/didymium metal is produced. The working temperature is slightly above the melting point of the metal/alloy and this allows the liquid metal to drop into the tungsten crucible positioned below the cathode. Where the ‘anode effect’ occurs, when the concentration of REO in the solution drops off and results in the production of perfluorocarbons (PFCs) from the anode, CF4 and C2F6 are emitted, which have a global warming potential 6 500 and 9 200 times of CO2, respectively (Green, 2007). PFCs production shoots up, current density is interrupted and metal production is stalled when the anode effect occurs.
Environmental considerations
The challenges of developing a successful REE molten-salt electrolysis method under European environmental standards led researchers at RWTH, Aaachen, to develop an automated new cell design (closed system). The main goal was to improve current molten salt technology by reducing energy losses, improving cost efficiency and reducing impurity interferences.
Multiple electrochemical techniques were used to determine the process window and recognise the mechanisms ongoing in the system, in order to both produce metal efficiently and prevent the production of PFCs. A breakthrough point of the project was the process automation, leading to a reduction of greenhouse gas production. Emission of these gases depends on oxide concentration and other process parameters, such as potential and current density, which were regulated by an installed controller, and a Fourier-transform infrared (FTIR) spectroscope was used to measure off-gases. As soon as the above-mentioned parameters reach critical values, the resulting emission of PFCs are detected, triggering an alarm and a dosing of REO, preventing the full anode effect. Through the EURARE project the fundamentals behind electrolysis of neodymium and didymium were investigated and the optimal process parameters were established. Furthermore, the fully automatised process control was installed based on critical potential and current values as well on off-gas concentrations leading to a high efficiency in metal production and reduction of PFC greenhouse gases.
The developed cell resulted in process parameter optimisation, automation, reduction of greenhouse gas emissions, and high purity metal production for Nd and didymium (Nd-Pr) metals. Elucidation of the process of didymium metal production was a significant breakthrough led by the EURARE project, as previously this information did not exist in the literature. Automation of the process has led to high metal production efficiency and reduction in PFC greenhouse gas production. The biggest advantage of the process is continuously produced metal with high purity, in comparison to metallothermic reduction which is batch process and the product requires further purification steps.
Electrodeposition of reactive metals
Alternatives to traditional high temperature molten salt electrolysis were also explored under the EURARE project. Ionic liquids have gained increasing attention in recent years, as electrolytes for the electrodeposition of metals more electropositive than hydrogen. The type of cation and anion changes several properties of the ionic liquids, such as melting point, chemical and thermal stability, ionic conductivity and broad electrochemical window.
Researchers from NTUA (Athens) used ionic liquids that melt at low temperature, or ‘room temperature ionic liquids’ (RTIL), for the electrodeposition of both La and Nd.